3 exciting ways that chemists constructed compounds this year
by Bethany Halford
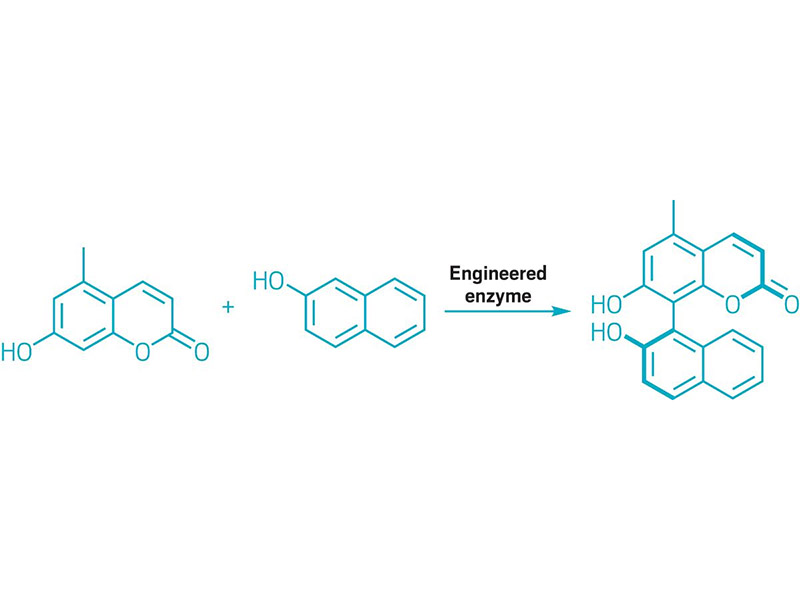
EVOLVED ENZYMES BUILT BIARYL BONDS
Scheme showing an enzyme-catalyzed biaryl coupling.
Chemists use biaryl molecules, which feature aryl groups tethered to one another by a single bond, as chiral ligands, materials building blocks, and pharmaceuticals. But making the biaryl motif with metal-catalyzed reactions, such as Suzuki and Negishi cross-couplings, typically requires several synthetic steps to make the coupling partners. What’s more, these metal-catalyzed reactions falter when making bulky biaryls. Inspired by enzymes’ ability to catalyze reactions, a team led by the University of Michigan’s Alison R. H. Narayan used directed evolution to create a cytochrome P450 enzyme that builds a biaryl molecule via oxidative coupling of aromatic carbon-hydrogen bonds. The enzyme weds aromatic molecules to create one stereoisomer around a bond with hindered rotation (shown). The researchers think this biocatalytic approach could become a bread-and-butter transformation for making biaryl bonds (Nature 2022, DOI: 10.1038/s41586-021-04365-7).

RECIPE FOR TERTIARY AMINES RELIED ON A LITTLE SALT
Scheme shows a reaction that makes tertiary amines from secondary ones.
Mixing electron-hungry metal catalysts with electron-rich amines typically kills the catalysts, so metal reagents can’t be used to build tertiary amines from secondary amines. M. Christina White and colleagues at the University of Illinois Urbana-Champaign realized they could get around this problem if they added some salty seasoning to their reactant recipe. By transforming secondary amines into ammonium salts, the chemists found they could react these compounds with terminal olefins, an oxidant, and a palladium sulfoxide catalyst to create myriad tertiary amines with a variety of functional groups (example shown). The chemists used the reaction to make the antipsychotic drugs Abilify and Semap and to transform existing drugs that are secondary amines, such as the antidepressant Prozac, into tertiary amines, demonstrating how chemists might make new drugs out of existing ones (Science 2022, DOI: 10.1126/science.abn8382).
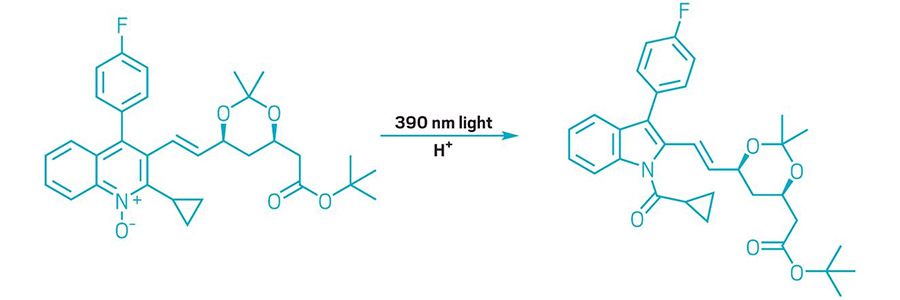
AZAARENES UNDERWENT CARBON CONTRACTION
Scheme shows a quinoline N-oxide transformed into an N-acylindole.
This year chemists added to the repertoire of molecular editing, which are reactions that make changes to the cores of complex molecules. In one example, researchers developed a transformation that uses light and acid to clip a single carbon out of six-membered azaarenes in quinoline N-oxides to form N-acylindoles with five-membered rings (example shown). The reaction, developed by chemists in Mark D. Levin’s group at the University of Chicago, is based on a reaction that involved a mercury lamp, which put out multiple wavelengths of light. Levin and colleagues found that using a light-emitting diode that emits light at 390 nm gave them better control and allowed them to make the reaction general for quinoline N-oxides. The new reaction gives molecule makers a way to remodel the cores of complex compounds and could help medicinal chemists looking to expand their libraries of drug candidates (Science 2022, DOI: 10.1126/science.abo4282).
Post time: Dec-19-2022

